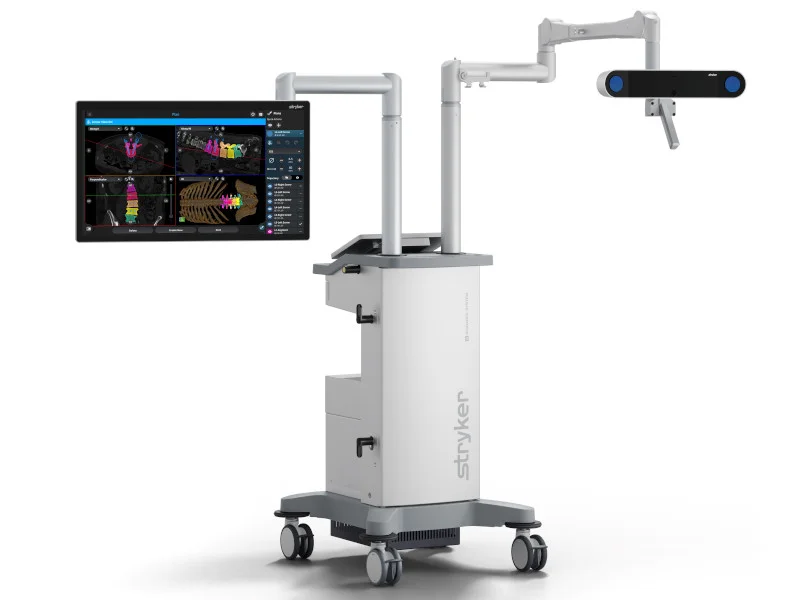
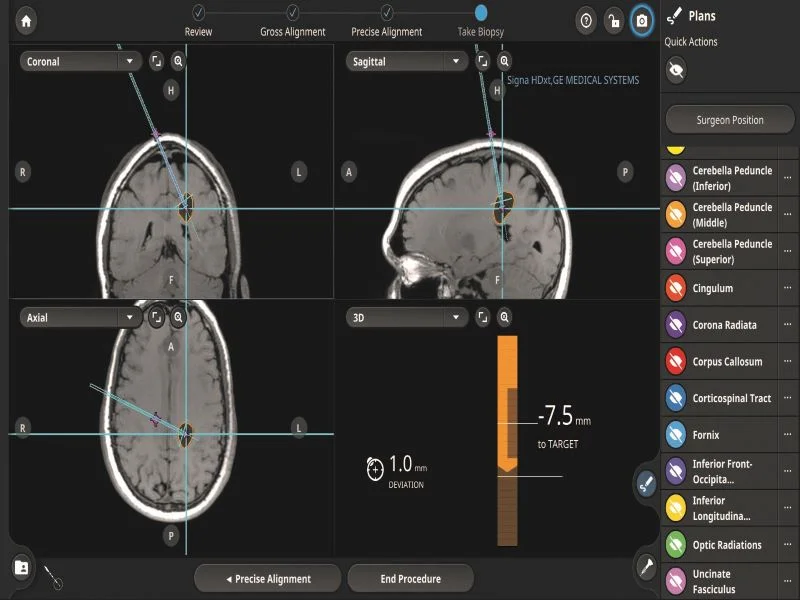
Stryker's Q Guidance System for cranial applications has received FDA clearance. The Cranial Guidance Software monitors and displays navigated instruments, displaying the instrument's orientation and position within patient images. This enables greater confidence and enhanced surgical precision. The system incorporates algorithmic processing, comprehensive guidance data, and an interface supporting greater efficiency and accuracy.
UEGroup is proud to have supported the UX design and research process behind the scenes, and we are looking forward to seeing the impact this imaging and guidance technology will have once it’s in surgeons’ hands! Check out Stryker's full announcement.
We have a long partnership with Stryker supporting platforms such as SpineMap Go, HipMap, and HipCheck. You can read our case studies on Hip Arthroscopy and Spine Navigation to learn more about our approach to advanced medical UX.